
 Glenn Seaborg was 26 when he heard that German scientists had split the atom.
Glenn Seaborg was 26 when he heard that German scientists had split the atom.
 Berkeley physicist Ed McMillan created the first “transuranic” element, neptunium, in 1940.
Berkeley physicist Ed McMillan created the first “transuranic” element, neptunium, in 1940.
 America’s atomic bomb program began after Albert Einstein warned President Roosevelt that Germany was attempting to build a bomb of its own.
America’s atomic bomb program began after Albert Einstein warned President Roosevelt that Germany was attempting to build a bomb of its own.
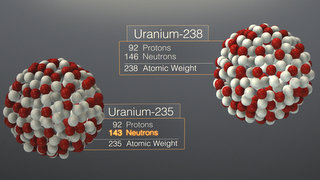 Seaborg’s discovery of plutonium was important because only one percent of natural uranium (U-235) could be split.
Seaborg’s discovery of plutonium was important because only one percent of natural uranium (U-235) could be split.
 Seaborg was newly married and just 30 years old when he was asked to join the Manhattan Project in Chicago.
Seaborg was newly married and just 30 years old when he was asked to join the Manhattan Project in Chicago.
 Seaborg and McMillan shared the 1951 Nobel Prize in chemistry for their discovery of the first two transuranic elements.
Seaborg and McMillan shared the 1951 Nobel Prize in chemistry for their discovery of the first two transuranic elements.
 Seaborg points to the element named for him: seaborgium.
Seaborg points to the element named for him: seaborgium.
The Atom Splits
Glenn Seaborg was a 26-year-old chemistry instructor at the University of California, Berkeley, when he heard the news that shook the scientific world: German scientists had split the uranium atom. This stunning discovery, announced in January 1939, had profound implications for a world at the brink of war. Right away, scientists and military planners wondered if the tremendous energy released during nuclear fission could be used to make a powerful new kind of weapon – the atomic bomb. But for Seaborg, the news of fission meant something else. The German discovery debunked physicist Enrico Fermi’s claim to have created artificial elements that were heavier than uranium, the last naturally occurring element in the Periodic Table. Suddenly, there was a second chance to be the first scientist to create “transuranic” elements.
Elements Beyond Uranium
Relying on Cal’s huge cyclotron to bombard uranium atoms with neutrons, one of Seaborg’s Berkeley colleagues, physicist Ed McMillan, soon discovered the first true transuranic – element 93: neptunium. It was a turning point in the search for the elements. Up to then, element hunters had been finding elements that already existed in nature. But from this point on, they would be creating new ones.
When McMillan was called away to work on the development of radar, Seaborg jumped at the chance to continue the work. In early 1941, he and graduate student Arthur Wahl created element 94: plutonium. And they had reason to believe it might be “fissionable” – that, like uranium, it could be split.
Could It Be Used In a Bomb?
By this time, at the urging of Albert Einstein and amid fears the Nazis might be building a bomb, President Roosevelt had authorized a modest research program into the possibility of weapon powered by the fission of uranium. One huge challenge loomed: Only about one percent of the world’s uranium is in a form that is easy to split: U-235. The other 99 percent is the relatively stable U-238. This is what made Seaborg’s discovery so important. “We knew early on that element 94 could be a big prize,” Seaborg later recalled. “If we could transform U-238 into a fissionable material, we would increase a hundredfold the amount of material usable for a bomb.”
In March 1941, Seaborg and physicist Emilio Segrè spent three days going through a tedious series of steps to produce a sample of plutonium large enough to test. They placed the sample in front of the cyclotron’s neutron window and had their answer almost immediately: They heard the “unmistakable kicks” of fission. “They knew immediately what the implications were,” explains Seaborg’s son and biographer, Eric Seaborg. “There was a large portion of uranium that could not be used in a bomb. What plutonium offered was a chance to turn all of that uranium 238 into a fissionable material.”
“Seaborg figured out how to take this uranium 238 and turn it into a new element, plutonium, which readily fissions,” says University of Maryland physicist Jim Gates.
The Bomb That Ended World War II
Nine months later, the Japanese attack on Pearl Harbor plunged America into war, and in April 1942, Seaborg reported to the University of Chicago to join the Manhattan Project. Just 30 years old, he was put in charge of a team of chemists responsible for developing a method for separating plutonium from other fission products in nuclear reactions. In 1943, banking on the process Seaborg’s team had developed, the U.S. government built a huge separation plant in Hanford, Washington. From Hanford came the pounds of plutonium that were needed for a bomb.
On July 16, 1945, at a desert site near Alamogordo, New Mexico, scientists from nearby Los Alamos conducted the first test of an atomic bomb, with a weapon made from plutonium. And on August 9, three days after a uranium bomb had destroyed the city of Hiroshima, a second plutonium bomb leveled the city of Nagasaki, finally bringing the war to an end.
Extending the Periodic Table
In 1951, Ed McMillan and Glenn Seaborg won the Nobel Prize in chemistry for their creation of the first two transuranic elements. By that time, Seaborg had already gone on to create more new elements – and engineered the last major realignment of the Periodic Table, moving plutonium and its neighbors (the “actinides”) to a new row at the bottom of the table, reflecting their similarity to the lanthanide series just above them. Since Seaborg and McMillan first ventured beyond uranium, more than 25 new elements have been added to the table – including one named for Seaborg himself. Today, in Germany, Russia and California, scientists continue working to create more new elements, using techniques like those Seaborg pioneered.
Want to Learn More?
To see the full Seaborg story, select Watch Online to go to pbs.org/mysteryofmatter and watch Episode 3. Or click on Buy to purchase the series DVD at ShopPBS. For more resources about Glenn Seaborg’s life and career, select Learn More.
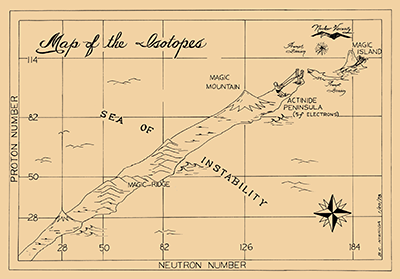 Article: The Search for the Island of Stability - Inspired by Seaborg, scientists across the world continue trying to create long-lived superheavy elements, extending the Periodic Table still further.
Article: The Search for the Island of Stability - Inspired by Seaborg, scientists across the world continue trying to create long-lived superheavy elements, extending the Periodic Table still further.