
 Dmitri Mendeleev as a young chemistry professor at St. Petersburg University
Dmitri Mendeleev as a young chemistry professor at St. Petersburg University
 Mendeleev’s first Periodic Table (1869)
Mendeleev’s first Periodic Table (1869)
 More than 50 years before Mendeleev published his table, German chemist Johann Döbereiner noticed a mathematical pattern among groups of three elements he called “triads.”
More than 50 years before Mendeleev published his table, German chemist Johann Döbereiner noticed a mathematical pattern among groups of three elements he called “triads.”
The Table’s Origins
The Periodic Table of the Elements is one of the most iconic images in all of science – a fixture in every chemistry classroom in the world. What many chemistry students don’t realize is that this ubiquitous chart had its origins in the struggles of a young Russian chemistry professor over one weekend in February 1869.
After years of scraping together a living, Dmitri Mendeleev had finally landed a coveted post at St. Petersburg University, and one of his responsibilities was to teach introductory chemistry. Dissatisfied with the existing Russian chemistry textbooks, Mendeleev set out to write his own. He had completed the first volume and the first two chapters of the second when he came to an impasse: He needed a way to organize the rest of the textbook, and to do that he felt he needed to organize the chemical elements themselves.
As he grappled with this challenge over that fateful weekend, he hit upon a way to organize the 63 elements that were then known. He arranged the elements in rows of increasing atomic weight but also kept their chemical properties in mind, skipping a spot or reversing two elements if necessary to make sure that elements with similar chemical properties fell into columns. By Monday evening, he had created a chart that would be the precursor of all the Periodic Tables that followed.
Hints of a Hidden Order Among the Elements
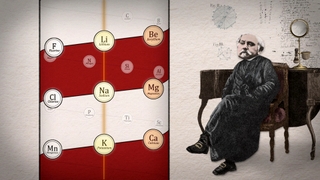
Mendeleev was not the first to seek order among the elements. The first hints of a hidden order actually dated back half a century to a German chemist named Johann Döbereiner. Chemists had long known that certain elements seemed to be related to each other – they had such similar properties that they were thought of as “chemical families.” What Döbereiner found was a curious mathematical pattern among some of these families: groups of three elements in which the atomic weight of the middle element was the average of the other two. For example, in a family known as the alkali metals, if you took the atomic weight of lithium, added it to the weight of potassium, and then divided by two, you’d get the atomic weight of another alkali metal, sodium.
Döbereiner called such groups-of-three "triads." He found another triad among the family known as the halogens: chlorine, bromine and iodine; and another among the alkaline earth metals: calcium, strontium and barium. First proposed in 1817, Döbereiner’s triads were a tantalizing clue that there was an underlying order to the elements. But it was an idea before its time. There simply wasn’t yet enough accurate information about atomic weights to develop a full-blown system of elements.
 Italian chemist Stanislao Cannizzaro cleared up the confusion about atomic weights at the 1860 Karlsruhe Congress.
Italian chemist Stanislao Cannizzaro cleared up the confusion about atomic weights at the 1860 Karlsruhe Congress.
 French geologist Alexandre de Chancourtois was the first to notice a periodic repetition in the properties of the elements. But he was largely ignored.
French geologist Alexandre de Chancourtois was the first to notice a periodic repetition in the properties of the elements. But he was largely ignored.
 British chemist John Newlands was ridiculed for suggesting an analogy between the elements and the musical scale.
British chemist John Newlands was ridiculed for suggesting an analogy between the elements and the musical scale.
 German chemist Julius Lothar Meyer began drafting his own Periodic Table five years before Mendeleev started his.
German chemist Julius Lothar Meyer began drafting his own Periodic Table five years before Mendeleev started his.
 One of the three elements whose properties Mendeleev accurately predicted was gallium, a metal so soft it melted in its discoverer’s hand.
One of the three elements whose properties Mendeleev accurately predicted was gallium, a metal so soft it melted in its discoverer’s hand.
 Using X-rays to study the elements, British physicist Harry Moseley discovered that each element is defined by its “atomic number” – the positive charge on its nucleus.
Using X-rays to study the elements, British physicist Harry Moseley discovered that each element is defined by its “atomic number” – the positive charge on its nucleus.
 Building on Moseley’s work, physicist Ernest Rutherford discovered the proton – the particle responsible for the positive charge on the nucleus of each element.
Building on Moseley’s work, physicist Ernest Rutherford discovered the proton – the particle responsible for the positive charge on the nucleus of each element.
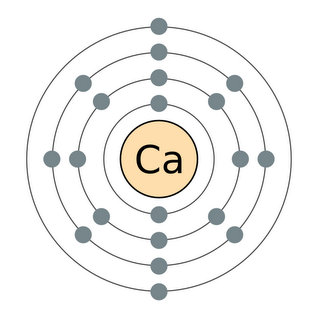 Electrons are arranged in discrete shells around each atom’s nucleus, with the outer electrons being the most reactive. All the elements in each column of the Periodic Table have the same number of electrons in their outer shells – and therefore have similar chemical properties. This is what makes the Periodic Table periodic.
Electrons are arranged in discrete shells around each atom’s nucleus, with the outer electrons being the most reactive. All the elements in each column of the Periodic Table have the same number of electrons in their outer shells – and therefore have similar chemical properties. This is what makes the Periodic Table periodic.

 Fifty-five new elements have been added to the Periodic Table since Mendeleev first drew it up.
Fifty-five new elements have been added to the Periodic Table since Mendeleev first drew it up.
 The discovery of argon by British scientists Lord Rayleigh and William Ramsay initially threatened the Periodic Table. But Ramsay’s discovery of four similar gases revealed that the table simply needed a new column for these inert elements: the noble gases.
The discovery of argon by British scientists Lord Rayleigh and William Ramsay initially threatened the Periodic Table. But Ramsay’s discovery of four similar gases revealed that the table simply needed a new column for these inert elements: the noble gases.
 American chemist Glenn Seaborg made the last major re-alignment of the Periodic Table, moving the “actinides” to a new row at the very bottom of the table.
American chemist Glenn Seaborg made the last major re-alignment of the Periodic Table, moving the “actinides” to a new row at the very bottom of the table.
 One of the many unusual forms of the Periodic Table
One of the many unusual forms of the Periodic Table
Previous Attempts to Arrange the Elements
Most chemists of the 19th century believed each element had its own unique kind of atom, and ever since the early 1800s they’d been working to determine how much an atom of each element weighed. That’s what distinguished on element from another, so it was critical to get these weights right. But determining an element’s atomic weight was fraught with difficulties, so by mid-century there were several competing systems of atomic weights – and widespread confusion among chemists. Hoping to sort out the mess, chemists organized their first-ever international meeting, held in Karlsruhe, Germany, in 1860. Out of the Karlsruhe Congress came a new, consistent set of atomic weights that made it possible to detect previously hidden mathematical patterns among the elements. Almost immediately, people begin looking for ways to arrange them.
One of the first to have a go at it was a French geologist named Alexandre de Chancourtois. In 1862, de Chancourtois listed all the known elements in the order of their atomic weights, then arranged them in a spiral along the outside of a cylinder, like the stripes on a barber pole. He found that elements with similar properties tended to line up vertically. The same family groupings Döbereiner had noticed were showing up here in what de Chancourtois called his Telluric Screw. Unfortunately, the journal that published de Chancourtois’ paper left out his diagram, making it hard for readers to grasp his spiral arrangement. Chemists paid no attention to the geologist’s idea, and the Telluric Screw sank without a trace.
The next to pursue the mysterious mathematical patterns among the elements was an English industrial chemist named John Newlands. In 1866, during a talk at the Chemical Society of London, Newlands pointed out that if the elements were arranged by atomic weight in rows of seven, they naturally fell into columns with similar chemical properties. Noting the resemblance to the musical scale, Newlands called this the Law of Octaves. But this idea, offered by a mere sugar chemist, struck a sour note with the academic chemists who heard his talk. They scoffed at his musical analogy and dismissed his ideas.
Another noteworthy attempt was made by chemist Julius Lothar Meyer of Germany, who published a table of elements that is similar in many ways to the one Mendeleev published a few years later. But Meyer’s 1864 table included only 28 of the known elements, leaving more than 30 unaccounted for. Meyer continued to improve his table and, by 1868, had drafted one that was nearly complete. But he had not published the table when Mendeleev set out to organize his textbook in February 1869 ... and ended up creating the Periodic Table we know today.
A Law of Nature
One reason Mendeleev gets the most credit for inventing the Periodic Table is that he alone, among all the “fathers” of the periodic system, had the courage of his convictions. Mendeleev believed his table was more than a convenient way to arrange the elements. He was sure he had discovered a law of nature: that the properties of the elements are determined by their atomic weights and vary in a regular, periodic way across the table. Mendeleev was so confident of his Periodic Law that in 1871 he took the bold step of making detailed predictions about the chemical properties of three yet-to-be-discovered elements for which he’d left room in his table. Within 15 years, all three elements (gallium, scandium and germanium) had been found, with properties almost exactly like those Mendeleev had predicted.
Atomic Number vs. Atomic Weight
When Mendeleev drew up his first Periodic Table, he relied heavily on the concept of atomic weight. Except for a few places where he had to skip a spot or invert two elements to keep them aligned with their chemical families, Mendeleev arranged the known elements in rows of increasing atomic weight, and he believed these weights determined their chemical properties. But a half-century later, British physicist Harry Moseley showed that the elements are actually arranged in the table in order of atomic number rather than atomic weight.
Up to then, “atomic number” had simply referred to the number of an element’s box in the Periodic Table. But Moseley showed it was more than a convenient label. Using a technique called X-ray spectroscopy, he showed that an element’s atomic number represented the amount of positive charge on its central core, the nucleus. Building on this work, Moseley’s mentor, physicist Ernest Rutherford, soon discovered the subatomic particle responsible for this charge: the proton.
Together, Moseley and Rutherford put the Periodic Table on a whole new footing. They showed that an element’s identity is determined not by its atomic weight but by the number of protons in it nucleus – its atomic number. Each element in the Periodic Table has one more proton in its nucleus than the element before it – without exception. Add a proton to an atom and you change its very identity.
Although Moseley showed that atomic number, not atomic weight, was the organizing principle underlying the Periodic Table, both numbers are still present in today’s table. An element’s atomic weight is the sum of the weights of all the subatomic particles its atoms contain. Most of the weight comes from the two kinds of particles that occupy the nucleus – the positively-charged protons and the neutrons, which have almost the same weight as the protons but no electric charge. Finally, a small amount of each atom’s weight comes from the third piece of the atom, the ultralight, negatively charged electrons, which are arranged in discrete shells around the nucleus.
What Makes the Periodic Table Periodic?
The Periodic Table is called “periodic” for the very reason Mendeleev noted in describing his law: The properties of the elements vary in a regular, predictable way, slowly changing as you move, element by element, across the table, and then repeating the same pattern in the rows below. As a result, all the elements in any one column of the table are similar to each other – but different from elements in the columns to the left and right.
Mendeleev knew that elements in each column had similar properties – that’s what made them members of the chemical families he used to organize the table in the first place. But he had no idea why these families existed. It took another half century for chemists and physicists to discover the underlying reason for periodicity: All the elements in a given column of the table have the same number of electrons in their outer shells. Since the outer electrons determine how atoms interact with each other, this means all the elements in that column will behave in more or less the same way.
A Changing Table
Seeing the Periodic Table up there on the classroom wall, fixed in plastic, it’s easy to think of the table as static. In fact, it is anything but permanent. The table has been changing constantly since Mendeleev first drew it up nearly 150 years ago, and it continues to evolve today. The main driver of this change is the discovery of new elements. The number of known elements has nearly doubled, from 63 in Mendeleev’s day to 118 today. To find out how and when the elements were discovered, click on the Timeline of Chemical Element Discoveries.
The fact that Mendeleev’s table has been able to accommodate all these new elements and remain essentially intact is a testament to the soundness of his thinking. But the table has faced its share of crises. In the 1890s, for example, British scientists Lord Rayleigh and William Ramsay discovered a new gas called argon that didn’t fit into the table. And a few years later, Ramsay discovered helium here on earth – 30 years after its spectrum was first detected in the sun. Helium, too, had no place in the table. For a time, it seemed Mendeleev’s carefully crafted edifice might come tumbling down.
But three years later, Ramsay discovered three similar gases: neon, krypton and xenon. It was clear that all these elements were members of a new chemical family that had gone undetected because they were all “inert” – they didn’t interact with other elements. Mendeleev responded by adding a new column to the far right of his table: the family of noble gases. These gases, initially seen as a threat to the table, turned out to be a vindication, proving the validity of Mendeleev’s periodic system.
Half a century later, American chemist Glenn Seaborg proposed another change in the table, moving six elements (actinium, thorium, uranium, protactinium, neptunium and plutonium) from positions they had occupied for years to a new row at the bottom of the table, reflecting their similarity to the lanthanide series just above them.
Seaborg’s “actinide” series resulted from new thinking, rather than new discoveries, but it enabled Seaborg and his team to go on and create many new artificial elements. In today’s table, the last naturally occurring element, uranium (92), is followed by 26 manmade elements. And in Germany, Russia and California, scientists continue trying to create still more new elements, using techniques like those Seaborg pioneered. With each new element they create, the Periodic Table will change again. Where it will end, no one knows.
A Table with Many Forms
Of the millions of Periodic Tables that hang in chemistry classrooms around the world, nearly all have the same basic rectangular shape: each element in its own box, arranged in orderly rows and columns. But in the century and a half since Dmitri Mendeleev first proposed it, people have re-imagined the table in scores of creative ways – from ribbon-like Möbius strips to block towers, triangles, spirals, helices, cylinders and even galaxies. This fantastic variety of forms is testament to the powerful hold the Periodic Table has had on the human imagination. For a tour of the many variants on the table, visit the Internet Database of Periodic Tables.
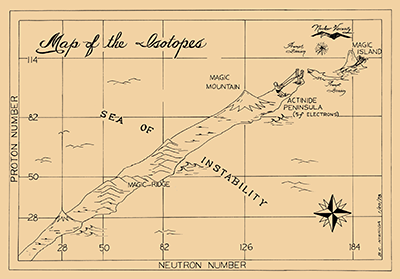 Article: The Search for the Island of Stability - Inspired by Seaborg, scientists across the world continue trying to create long-lived superheavy elements, extending the Periodic Table still further.
Article: The Search for the Island of Stability - Inspired by Seaborg, scientists across the world continue trying to create long-lived superheavy elements, extending the Periodic Table still further.